CLINICAL INVESTIGATION • A Doppler Echocardiographic Pulmonary Flow Marker of Massive or Submassive Acute Pulmonary Embolus
Source: J Am Soc Echocardiogr 2019;32:799-806
BACKGROUND
The reported in-hospital mortality of patients with massive PE (MPE) varies from 25% to 50%, and the mortality for submassive PE (SMPE; defined as the presence of right ventricular [RV] dysfunction without systemic hypotension) ranges from 3% to 15%. The mortality associated with low-risk, or subsegmental PE (SSPE) is < 5%.
Traditionally, the most frequently used noninvasive imaging modality for the diagnosis of acute PE include the multidetector row computed tomographic angiography (CTA) and, to a lesser extent, ventilation-perfusion lung scanning
The role of echocardiography in the workup of suspected PE has historically been supportive and primarily reserved for the detection of RV strain or dysfunction in unstable patients. The purpose of this study was to provide a head-to-head comparative assessment on the performance of several echocardiographic variables in a cohort of patients with MPE or SMPE identified following CTA for suspected PE.
METHODS
This was a retrospective study of all cases of contrast CTA performed for suspected PE at a tertiary care hospital.
Inclusion criteria included:
- Undergo transthoracic echocardiography within 48 hours of computed tomographic angiographic diagnosis of PE.
- complete echocardiographic examinations, including interpretable pulsed-wave (PW) Doppler signals across the RVOT, measurable tricuspid regurgitation jet Doppler signals, and discernible endocardial border definition of the right ventricle.
Exclusion criteria included:
- Technically suboptimal-quality PW Doppler studies with sample volumes placed at or distal to the pulmonary valve or poorly aligned to the direction of RVOT flow
- Patients with more than moderate valvular heart disease,
- Known history of PE
- Established chronic thromboembolic pulmonary hypertension
- Preexisting pulmonary hypertension, regardless of etiology, were excluded from this study.
Imaging:
Contrast-enhanced computed tomographic scans were performed on multidetector 64-slice scanners using a standardized PE timing protocol.
RV strain, dilation, or dysfunction was deemed present if four-chamber RV diameter divided by left ventricular diameter was > 0.9 on CTA. The diagnosis of PE was confirmed when thromboemboli were visualized in an at least segmental pulmonary artery on CTA.
Patients with PE were stratified according to 2011 definitions recommended by the American Heart Association scientific statement. MPE was defined as acute PE with sustained hypotension (systolic blood pressure < 90 mm Hg for >15 min or requiring inotropic support, not due to other causes (e.g. arrhythmia, hypovolemia, sepsis, or left ventricular dysfunction, etc.).
SMPE was defined as acute PE without systemic hypotension (systolic blood pressure < 90 mm Hg) but with RV dilation, defined as RV diameter divided by left ventricular diameter > 0.9 on CTA (in the four-chamber view). SSPE was defined as acute PE and the absence of clinical markers of adverse prognosis that define MPE or SMPE.
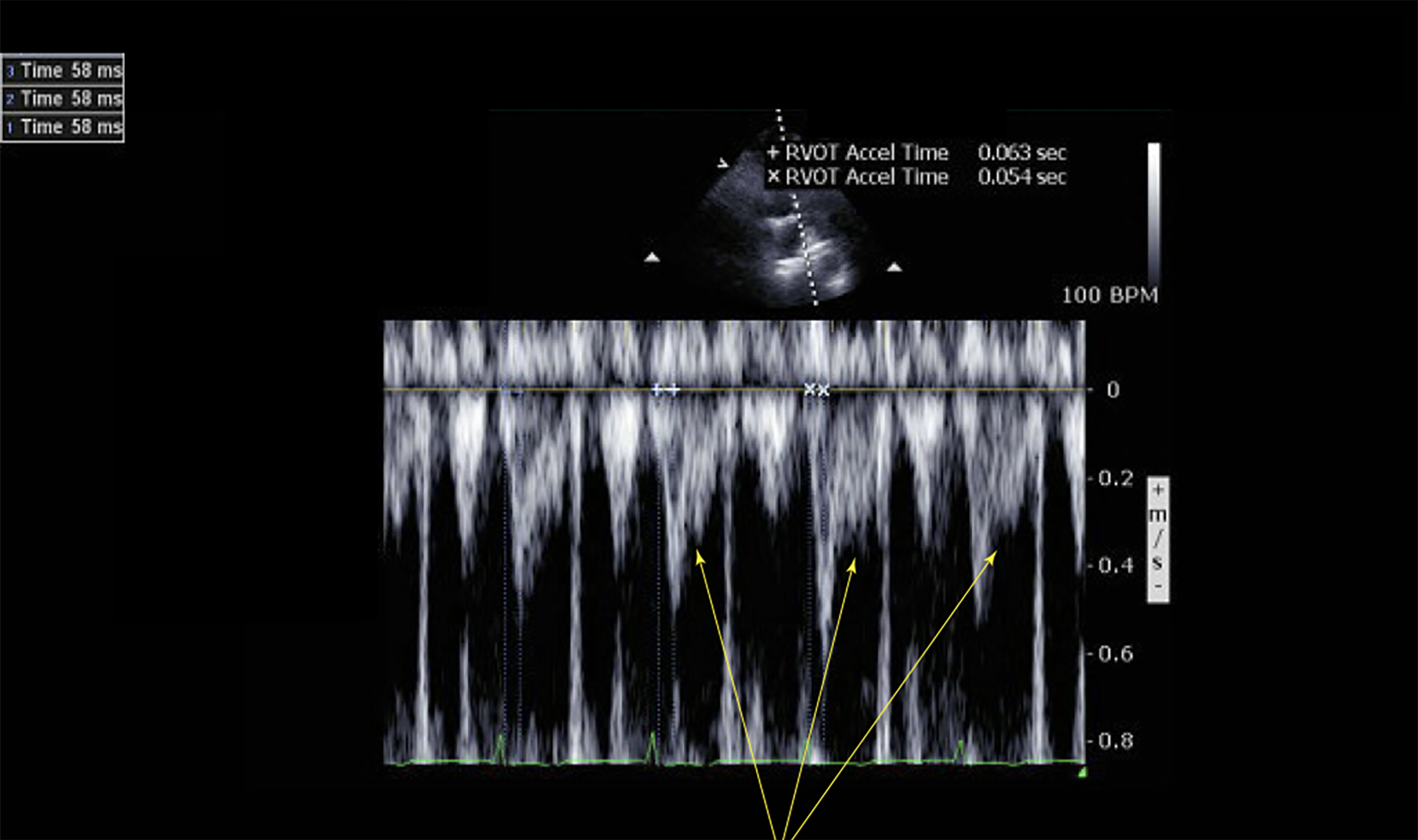
Echocardiographic variables were analyzed from transthoracic echocardiograms. PW Doppler interrogation of the RVOT was performed from the parasternal short-axis view at the level of the aortic valve or from the subcostal short-axis view, with the sample volume placed approximately 0.5 cm proximal to the pulmonic valve. The ‘‘early systolic notching’’ (ESN) pattern (spike and dome morphology) was visually assessed and deemed present if the Doppler envelope exhibited a narrow peaked initial wave (spike) with early deceleration of the RVOT envelope producing a sharp notch within the first half of systole, followed by a second Doppler wave (dome) that was more curvilinear in appearance.
Midsystolic notching was defined as a distinct notch falling within the second half of the systolic ejection period or, if the nadir occurred closer to the end of ejection, dividing the flow profile into two distinct peaks. Notch morphology was best appreciated when the Doppler beam was aligned parallel to RVOT outflow, with the PW sample volume placed at the appropriate location at sweep speeds of 50 to 100 mm/sec.
Ejection time was measured in milliseconds from the beginning to the end of the RVOT Doppler envelope. Acceleration time (AT) was measured in milliseconds as the time to peak velocity of the RVOT envelope measured from the beginning of ejection. Deceleration Time was measured from the peak Doppler velocity to the end of ejection.
The 60/60 sign was deemed present if RVOT AT was < 60 msec in the presence of a tricuspid peak systolic gradient > 30 mm Hg but < 60 mm Hg. Regional pattern of RV dysfunction consistent with McConnell’s sign was defined as akinesia of the mid free wall visualized along with preserved apical contractility.
RESULTS
526 out of 5,152 (10.2%) patients had positive results for PE (including MPE or SMPE and SSPE). After initial screening criteria, a total of 260 patients from this group were short-listed for further analysis. Upon further screening and application of exclusion criteria (limited studies, RVOT PW Doppler data not recorded, or Doppler data recorded but technically inadequate for interpretation moderate or greater valvular disease), a total of 187 patients with PE qualified and were included in the final analysis. The reference group included a total of 90 patients without PE who met our selection criteria.
ESN was observed in 92% of patients with MPE or SMPE, 2% of those with SSPE, and in no patients without PE. There was good interobserver agreement in the identification of ESN, with 96.7% agreement (k = 0.93, P < .001). Among patients with SSPE, systolic notching was predominantly midsystolic (19%) and less likely early systolic (2%).
Among patients with MPE or SMPE, systolic notching was predominantly early systolic (92%) and less likely midsystolic (1%). No systolic notching was observed in all control subjects (100%), 79% of patients with SSPE, and 7% of patients with MPE or SMPE.
Identification of the ESN pattern on echocardiography demonstrated good to excellent predictive ability for MPE or SMPE, with sensitivity of 92%, specificity of 99%, positive predictive value of 98%, negative predictive value of 96%, and area under the ROC curve of 0.96.
When compared with the widely recognized McConnell’s sign, these findings suggest superior predictive ability – in this study, the McConnell’s sign had a sensitivity of 52%, specificity of 97%, positive predictive value of 90%, negative predictive value of 82% and area under the ROC curve of 0.75.
The 60/60 sign yielded sensitivity of 51%, specificity of 96%, positive predictive value of 93%, negative predictive value of 70% and area under the ROC curve of 0.74.
CONCLUSION
The authors concluded that in patients with suspected acute PE, the pulmonary Doppler flow pattern of ESN has potential clinical utility for the detection of MPE or SMPE.
They commented on the fact that echocardiography has traditionally been relegated to a supportive role in the evaluation of patients with suspected PE and used primarily to assess RV size and function in hemodynamically unstable patients.
Other echocardiographic parameters described in the literature – McConnell’s sign, the 60/60 sign, paradoxical septal motion, and RV dilation – can be helpful and supportive, but lack the desired sensitivity, specificity, or negative predictive value (reported to be about 40%–50%) to be incorporated as first-line options in evaluation algorithms for suspected PE
In contrast, this study suggests that the simple visual assessment of RVOT Doppler morphology offers potentially insightful information on the coupling dynamics between the right ventricle and the pulmonary vasculature.
 English
English
 Español
Español